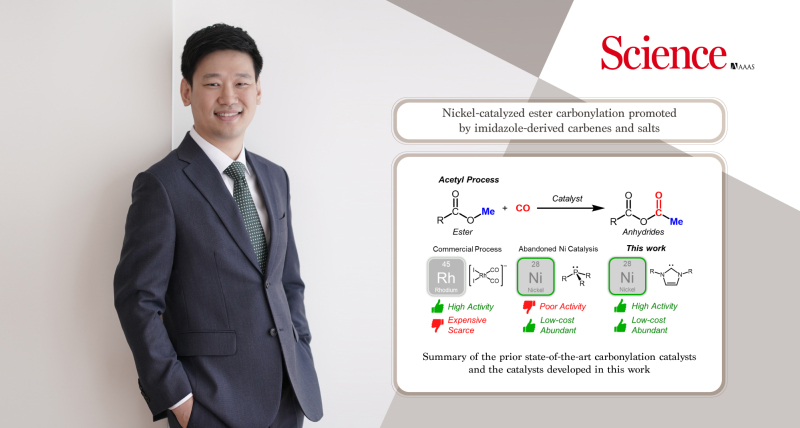
In a groundbreaking development set to transform the chemical industry on a global scale, Professor Changho Yoo in the Department of Chemistry at UNIST has successfully developed a highly efficient and stable carbonylation catalytic reaction using nickel catalysts. This revolutionary achievement, recently published in the esteemed journal Science on November 17, offers a promising alternative to the widely used rhodium catalysts in the acetyl process.
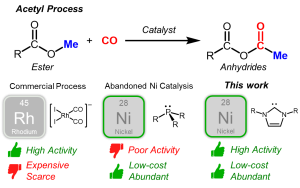
Carbonylation is a crucial reaction involved in the conversion of methanol to acetic acid and ester compounds to organic acid anhydride. With over 13 million tons of acetyl compounds produced annually worldwide, this reaction plays a vital role in the chemical industry.
Traditionally, precious metals such as rhodium and iridium have been employed as catalysts in the commercial carbonylation process. However, these metals present challenges due to their scarcity, high cost, toxicity, and environmental impact. In this groundbreaking research, Professor Yoo harnessed the abundant nickel transition metals as a sustainable and cost-effective alternative.
The existing nickel catalysts had limited catalytic activity, leading to discontinued industrial attempts since the 1970s. Professor Yoo’s research successfully addressed this limitation by employing an imidazole-based N-heterocyclic carbene ligand, replacing the conventional phosphine ligands used in traditional nickel catalysts. The carbene ligand demonstrated superior stability and activity, exhibiting comparable performance to the rhodium catalysts currently used in commercial applications.
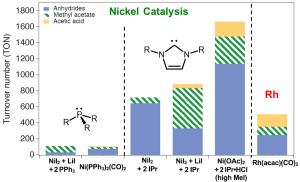
“We have developed a nickel catalyst that matches the activity level of the existing rhodium catalyst,” noted Professor Yoo. “This achievement is remarkable as it paves the way for the practical use of nickel catalysts in industrial settings.”
The research was conducted by Professor Yoo within the research team, led by Professor Alexander J. M. Miller at the University of North Carolina at Chapel Hill, with support and collaboration from the Eastman Chemical Company.
This groundbreaking development not only offers a sustainable alternative to precious metal catalysts but also holds the potential to revolutionize the chemical industry by enabling the widespread adoption of nickel catalysts. The abundance, low cost, and reduced environmental impact of nickel make it an attractive option for large-scale industrial applications. As this research breakthrough progresses towards commercialization, it is expected to drive significant advancements in the carbonylation process and open up new avenues for sustainable and efficient chemical production.
Journal Reference
Changho Yoo, Shrabanti Bhattacharya, Xin Yi See, et al., “Nickel-catalyzed ester carbonylation promoted by imidazole-derived carbenes and salts,” Science, 382, 815 (2023).