A team of researchers, led by Professor Chae Un Kim from the Department of Physics at UNIST, has successfully tracked the catalytic pathway of Carbonic Anhydrase II (CAII), an enzyme that converts carbon dioxide (CO2) into bicarbonate. This achievement was made possible through the use of a novel technique, which enables the construction of a molecular movie depicting the CAII catalytic pathway. The findings of this study are expected to shed new light on the role of water molecules in enzyme catalysis, ultimately contributing to the development of new drugs and bio-inspired catalysts.
CAII is a protein catalyst that plays a crucial role in converting CO2 into bicarbonate ions, which can easily dissolve in bloo The enzyme’s active site is where this conversion takes place, with CO2 binding and being converted into bicarbonate at an astonishing rate of over one million times per second. However, the intermediate steps of this reaction have long been considered impossible to observe directly due to their extremely fast pace.
To overcome this challenge, the research team used a proprietary molecular movie technology to capture the entire reaction process, from the binding of CO2 to the release of bicarbonate. By analyzing the X-ray crystal structure of the enzyme at various temperatures, the team discovered that the rearrangement and replacement of water molecules in the active site are the key factors determining the rate of product release.
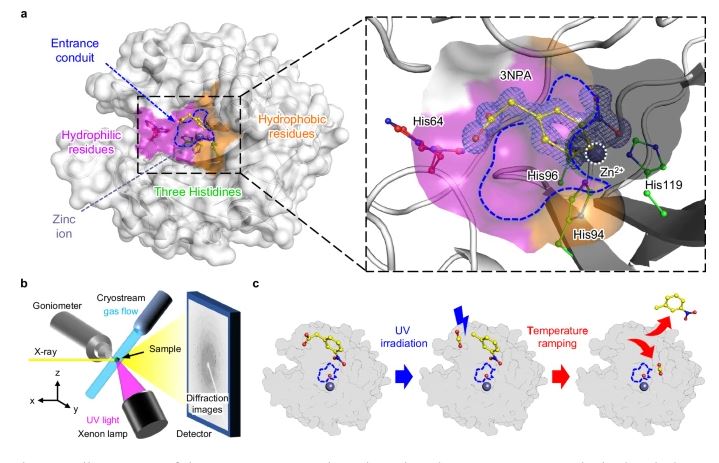
The reported technique involves a multi-step process. First, the enzyme is cooled to a temperature of -183°C. Then, a photosensitive substrate (3NPA) is introduced, which supplies carbon dioxide to the active site when exposed to ultraviolet light. The team then incrementally increases the temperature to -73°C, capturing the structural changes at each stage using X-ray crystallography. By reconstructing these structural data in chronological order, the researchers created a molecular slow-motion movie that reveals the intricate details of the enzyme’s catalytic process.
According to Jin Kyun Kim, the first author of the study, “We were able to directly observe the intermediate state of the reaction at the atomic level, which appears only in the temperature range of -113°C to -93°C. This is the first time that such a rapid enzymatic reaction has been structurally captured.”
Professor Kim added, “The newly discovered principle can be applied not only to protein engineering and drug development but also to the design of bio-inspired catalysts that precisely control water molecules.”
This breakthrough study, published in the prestigious journal, Nature Communications on May 12, 2025. This study was supported by various organizations, including the Samsung Future Technology Development Project, the National Research Foundation (NRF) of Korea, and the Ministry of Science and ICT (MSIT).
Journal Reference
Jin Kyun Kim, Seon Woo Lim, Hannah Jeong, et al., “Fast product release requires active-site water dynamics in carbonic anhydrase,” Nature, (2025).