A research team, led by Professor Jaeheung Cho from the Department of Chemistry at UNIST has unveiled the reaction mechanism of cobalt(III)-based metal complexes with nitrile substances, paving the way for potential new drug development.
In this study, the team investigated the mechanism of nitrile activation in biomimetic compounds utilizing cobalt(III), highlighting the significant impact of metal spin states on reaction activity. Their findings demonstrate that even small modifications to metal properties can profoundly influence the speed and outcomes of chemical reactions.
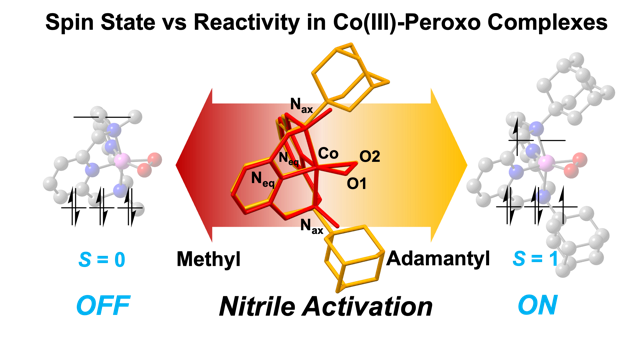
To examine how nitriles interact with cobalt compounds, the researchers employed a structure known as the ‘Macrocyclic Pyridinophane System,’ which enables adjustments in cobalt compound structures. Notably, compounds featuring larger adamantyl groups demonstrated enhanced nitrile activation reactions.
In contrast, compounds with smaller methyl groups showed no reactivity. This difference is attributed to changes in the metal’s spin states, which depend on the size of the functional group, resulting in distinct reactivity profiles.
Nitriles are widely used in pharmaceuticals and pesticides but often present challenges in reactivity. The team confirmed that cobalt(III)-peroxo species can react with nitriles at room temperature to form a specific compound, which shows promise as a potential anticancer agent.
“We successfully synthesized cobalt(III)-peroxo species with varying spin states by modifying the three-dimensional configuration of the ligands, revealing a close relationship with nitrile reactivity,” said first author Seonghan Kim. Professor Cho added, “Controlling the spin state of cobalt(III)-peroxo species is of significant academic importance and could pave the way for new developments in metal catalysts.”
This collaborative research involved Professor Jana Roithová from Radboud University in the Netherlands and Professor Sunggi Lee from DGIST. The findings, supported by the National Research Foundation of Korea (NRF), the Ministry of Science and ICT (MSIT), and the Ministry of Trade, Industry and Energy (MOTIE), were published online in the Journal of the American Chemical Society (JACS) on July 20, 2024.
Journal Reference
Seonghan KimYuri LeeGuilherme L. Tripodi, et al., “Controlling Reactivity through Spin Manipulation: Steric Bulkiness of Peroxocobalt(III) ComplexesArticle link copied!,” JACS, (2024).