A research team, affiliated with UNIST has successfully developed a new porous material for the efficient separation of deuterium, a primary fuel for nuclear fusion. The team, jointly led by Professors Wonyoung Cho and Hyunchul Oh in the Department of Chemistry at UNIST, announced this breakthrough online in the January 2025 issue of Angewandte Chemie International Edition, a leading chemistry journal.
Metal-organic frameworks (MOFs) are a form of porous and crystalline materials constructed by the formation of chemical bonds between metal ions and organic ligands. A MOF separates the heavy hydrogen isotope deuterium from ordinary hydrogen, just like grain sieves that detect millet and other grains for screening and grading.
The newly-developed MOF exhibits remarkable efficiency in deuterium separation, even at temperatures above the liquefied natural gas (LNG) liquefaction temperature (111K, -162.15°C). This is a significant improvement over the traditional method, which requires much colder cryogenic temperatures below 20K (-253.15°C).
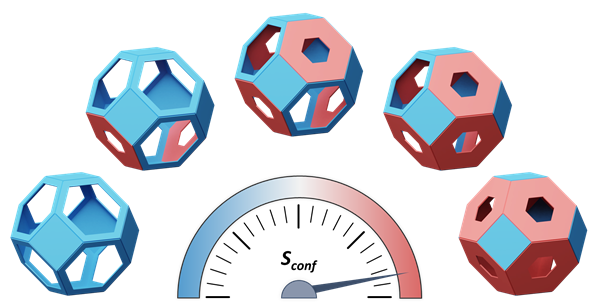
The team employed an entropy-driven design strategy to create this new material. By mixing different organic ligands, similar to creating a cocktail, they increased the structural disorder, or entropy, within the MOF. This high-entropy state optimized the process of quantum sieving, which separates hydrogen and deuterium based on their differing diffusion rates.
Quantum sieving exploits the differences in diffusion rates between hydrogen and deuterium. This results in a higher proportion of narrow pores in the framework, thereby improving the efficiency of the quantum sieving. The team confirmed this through X-ray diffraction and hydrogen isotope breakthrough experiments.
According to Professor Cho, “This study marks the first application of high-entropy porous materials for gas separation and adsorption, confirming the potential of entropy-based design.” He adds, “We hope this technology will contribute to the use of clean energy resources and the development of future energy technologies.”
The research team included Joohan Nam as the first author, alongside contributions from Changheon Cho, Yeongjin Kim, Yejin Hong, Sohyeon Lee, Sungyeop Jung, and Minji Jung. This study has been supported by the Ministry of Science and ICT (MSIT), the National Research Foundation of Korea (NRF), the Information and Communication Planning and Evaluation Institute (IITP), and UNIST.
Journal Reference
Joohan Nam, Changhyeon Cho, Sungyeop Jung, et al., “High-Entropy Zeolitic Imidazolate Frameworks for Dynamic Hydrogen Isotope Separation,” Angew. Chem. Int. Ed.,(2024).