A research team, led by Professor Ja-Young Ryu from the Department of Chemistry at UNIST has developed a carrier that combines metal-organic framework (MOF) nanoparticles with antibodies. This innovative carrier can accurately target specific cancer cells. Antibody proteins are attached to the surface of MOF particles, enhancing bio-environmental safety by reducing unnecessary interactions with non-cancerous cells.
MOF nanoparticles are composed of metal clusters and organic structures. Various combinations can be formulated to create particles with diverse functionalities, including biocompatibility, heat sensitivity, and light sensitivity. However, immune cells can eliminate or aggregate the defects and reactive sites present on the particle surface.
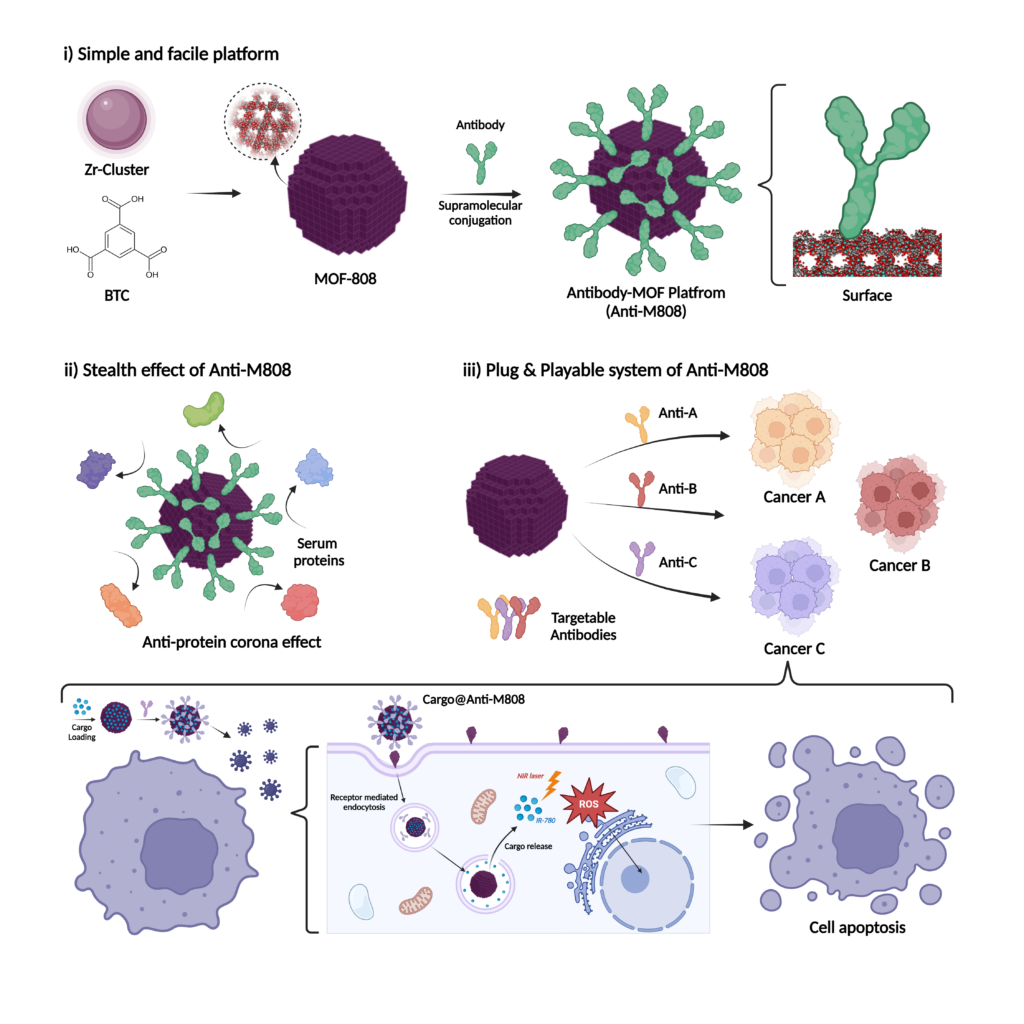
Addressing these challenges, the research team successfully conjugated antibody proteins to the MOF nanoparticles. This modification allows for precise identification of target cancer cells while minimizing unwanted reactions. The attachment of the antibody proteins does not induce significant chemical changes, thereby streamlining the process of complex formation.
The antibody-MOF complexes can target a wide variety of cancer cells. The antibody proteins serve as a protective layer, reducing adhesion to other proteins within the biological environment. Experimental results have demonstrated the efficacy of this complex.
Professor Ryu stated, “The method developed in this study has secured high versatility and stability as a drug carrier by attaching actual antibody proteins to MOF nanoparticles.”
The findings of this research have been published in ACS Nano on June 7, 2024. The research was conducted in collaboration with Professor Myoung Soo Lah in the Department of Chemistry at UNIST and Professor Sang Kyu Kwak from Korea University, and it received support from the National Research Foundation of Korea (NRF) under the Ministry of Science and ICT (MSIT).
Journal Reference
Jun Yong Oh, Batakrishna Jana, Junmo Seong, et al., “Unveiling the Power of Cloaking Metal–Organic Framework Platforms via Supramolecular Antibody ConjugationArticle link copied!,” ACS Nano, (2024).