The research groups of Professors Sung You Hong and Jan-Uwe Rohde of the UNIST Chemistry Department have developed a one-step synthetic method to access ‘nitrogen-containing polyaromatic hydrocarbons’ from simple azines, which have applications in chemical science, polymer chemistry, and energy industry. The research results were published in Nature Communications.
Polycyclic arenes have received significant attention not only in academia but also in industry due to their controllable chemical, electrochemical, and physical properties. The preparation of such materials can be achieved in a top-down manner from bulk materials with low operability or in a bottom-up approach from molecular building blocks with precise control of molecular size and structure.
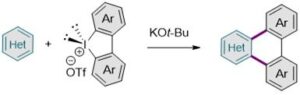
The research team developed a C–C bond formation process that involves a radical chain reaction (See Figure 1). C–H arylation typically requires expensive transition metal catalysts, however, this newly developed synthetic method provides a straightforward route to afford ‘nitrogen-containing polyaromatic hydrocarbons’ without the need for any pre-treatment processes and expensive transition metal catalysts. In addition, the team conducted mechanistic studies through chemical and electron paramagnetic resonance (EPR) spectroscopic analyses.
Figure 1. The iodanyl-Minisci-type radical approach, presented by the research team.
Dr. Jae Bin Lee and doctoral students Seo Yeong Jeong and Ji Hwan Jeon said “Without a use of a transition metal catalyst, N-containing polyaromatic hydrocarbons were directly synthesized from azines using diaryliodonium salts and KOt-Bu. This direct bottom-up approach introduces an innovative method to construct nitrogen-bearing nanographenes, while avoiding tedious synthetic operation steps” (See Figure 2).
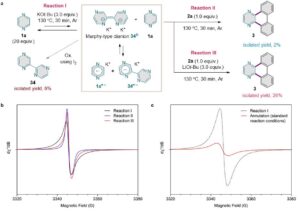
Figure 2. EPR spectroscopic studies.
Doctoral student Gun Ha Kim, who conducted the spectroscopic analysis, said “Analysis of in situ generated organic electron donors using EPR spectroscopy (See Figure 3) allowed an insight into a plausible reaction mechanism of this radical chain reaction”.
Professor Hong mentioned that this study introduced a unique approach to activate inert simple azines via iodanyl Minisci-type reactions. Professor Rohde commented that this work nicely showed how a collaboration of research groups with complementary expertise in synthesis and mechanistic analysis can inform the development and understanding of new reactions.
This work has been supported by the grants from the ERC SMILE Center, the Electronics and Telecommunications Research Institute (ETRI), and UNIST.
Journal Reference
Jae Bin Lee, Gun Ha Kim, Ji Hwan Jeon, et al., ‘Rapid access to polycyclic N-heteroarenes from unactivated, simple azines via a base-promoted Minisci-type annulation.’ Nat Commun., (2022). DOI: 10.1038/s41467-022-30086-0