A research team, jointly led by Professor Sung You Hong and Professor Jan-Uwe Rohde has unveiled a novel method for synthesizing allenes—a series of compounds integral to drug development and synthetic chemistry—without relying on hazardous, highly reactive chemicals.
Allenes exhibit a distinctive structure in which carbon atoms are double-bonded, making them essential for various applications in medicinal chemistry. The new synthesis method utilizes more stable organic halides instead of unstable organometallic compounds, enhancing safety in chemical reactions. Organic (pseudo) halides are compounds in which halogen elements, such as bromine and iodine, are covalently bonded to organic materials, allowing for more predictable reactivity compared to previously used alternatives.
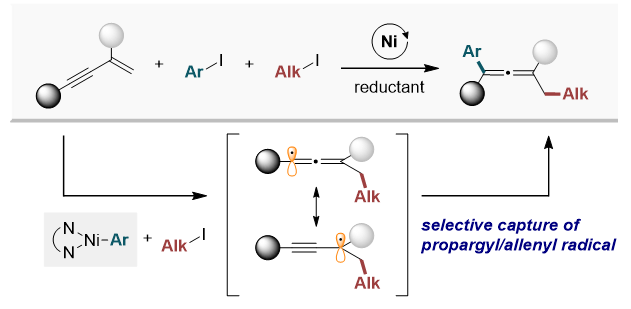
Under nickel-catalyzed reduction conditions, the research team successfully synthesized an allene compound with a consistent structure by sequentially combining three components: △ 1,3-enyne, △ alkyl iodide, and △ aryl iodide. Importantly, this method produced only the desired compound, avoiding the formation of irregular structures.
This process showcases excellent chemo- and regioselectivity in allene formation, enabling the efficient synthesis of complex allene compounds. Chemoselectivity ensures that only the targeted chemical reaction occurs, while regioselectivity allows for precise control over where this reaction takes place within the molecule.
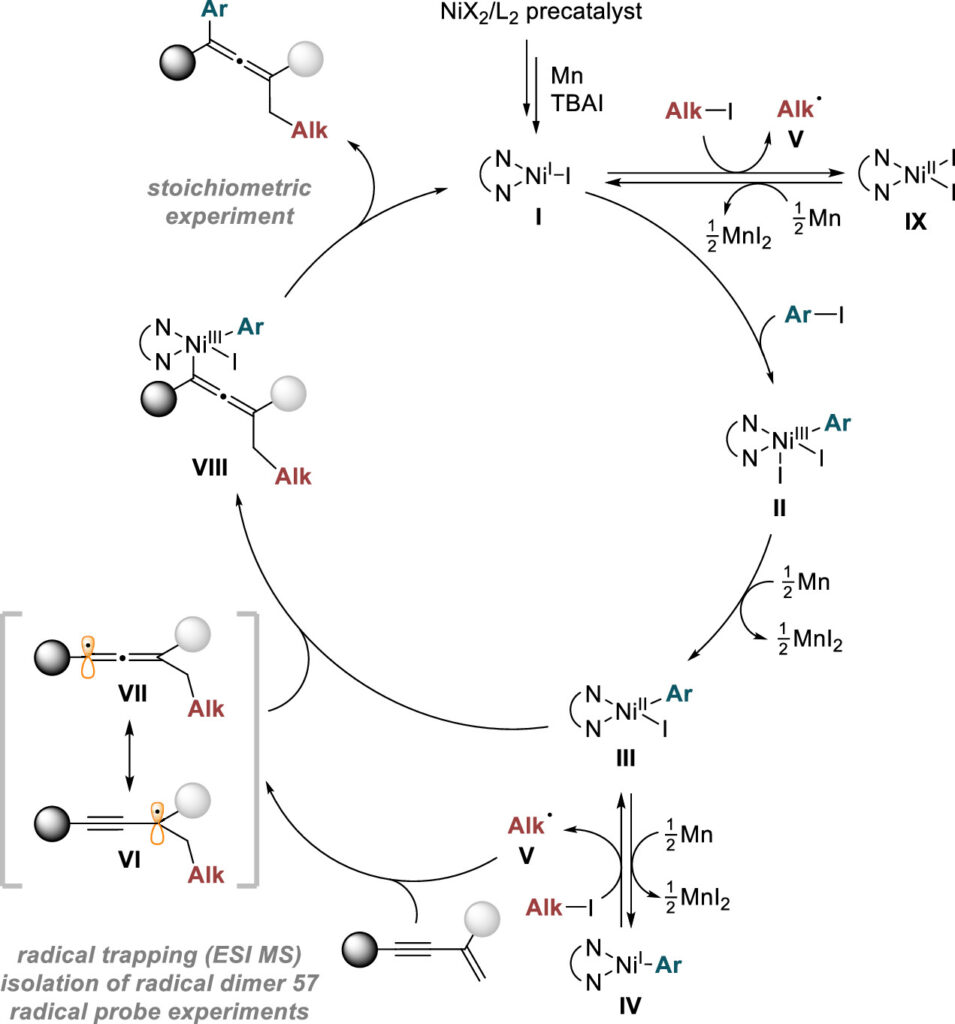
The research team’s approach to multi-component reactions—where three or more compounds are combined simultaneously—has proven to be a valuable synthetic pathway. They successfully extended this method to create more complex structures and demonstrated a broad range of functional group tolerance with various allene compounds, establishing the method’s stability with substances possessing diverse chemical properties.
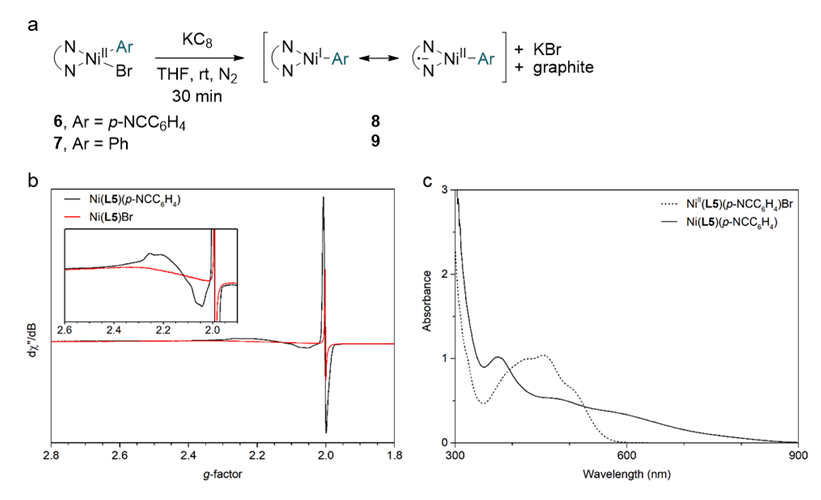
“Our research has revealed that reduced nickel complexes play a crucial role in the key stages of this reaction,” noted first authors Ji Hwan Jean and Gun Ha Kim. “We directly identified and studied nickel organometallic complexes, which are known for their instability and high reactivity.”
Professor Hong commented, “We can now synthesize complex allene compounds in a safe and straightforward manner,” adding, “This opens up new possibilities in the field of synthetic chemistry.”
Professor Rohde expressed optimism about future applications, stating, “A deeper understanding of these catalysts will pave the way for the development of coupling reactions that link two compounds to form novel entities.”
The findings of this research have been published in the online version of ACS Catalysis on May 28, 2024. The study was supported by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Ji Hwan Jeon, Gun Ha Kim, Ho Seung Lee, et al., “Chemo- and Regioselective Nickel-Catalyzed Reductive 1,4-Alkylarylation of 1,3-Enynes through an L2NiAr IntermediateArticle link copied!,” ACS Catalysis, (2024).