A joint research team, affiliated with UNIST has unveiled a novel electrolyte additive that could enable a long lifespan and fast chargeability of high-energy-density lithium-ion batteries (LIBs).
Published in the February 2021 issue of Nature Communications, this research has been carried out by Professor Nam-Soon Choi and Professor Sang Kyu Kwak in the School of Energy and Chemical Engineering, in collaboration with Professor Sung You Hong in the Department of Chemistry at UNIST. It has also been participated by Professor Jaephil Cho in the School of Energy and Chemical Engineering at UNIST.
As the demand for large-capacity batteries (i.e., EV batteries) increases, efforts are actively underway to replace the conventional lithium-ion battery electrodes with large-capacity materials, such as silicon or high-nickel. Although silicon is an attractive anode material to improve the energy density of LIBs, they exhibit poor mechanical strength due to volumetric expansion during charging and discharging. High-Ni cathode materials also suffer from poor chemical stability.
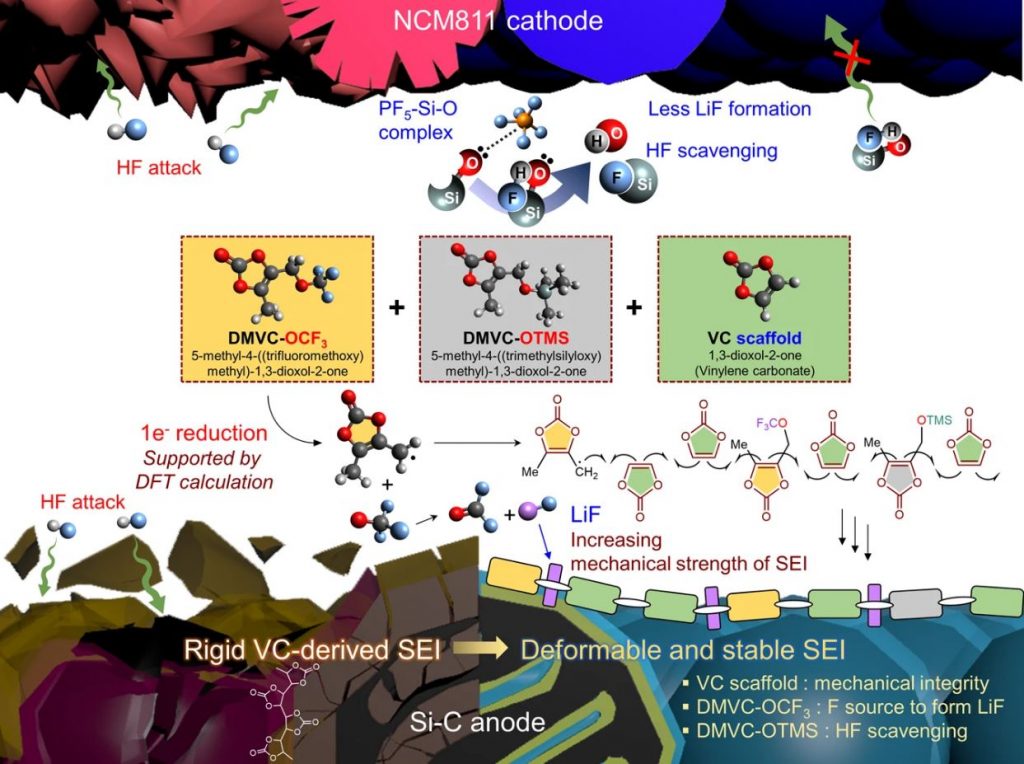
Figure 1. Incorporation of DMVC-OCF3 and DMVC-OTMS in the VC scaffold leads to the creation of a flexible and robust SEI on the Si–C anode. DMVC-OTMS scavenges HF and deactivates PF5, resulting in the compositional and structural stability of the interfacial layers on the electrodes. The Me (−CH3) moiety bonded to the VC scaffold provides ion channels, providing space for Li-ion transport in the SEI.
In the study, the research team demonstrated that the creation of a stable and spatially deformable solid electrolyte interphase (SEI) on a high-capacity Si–C anode could tolerate the inevitable volume changes induced by the lithiation of Si and could enable a long lifespan and fast chargeability of high-energy-density LIBs.
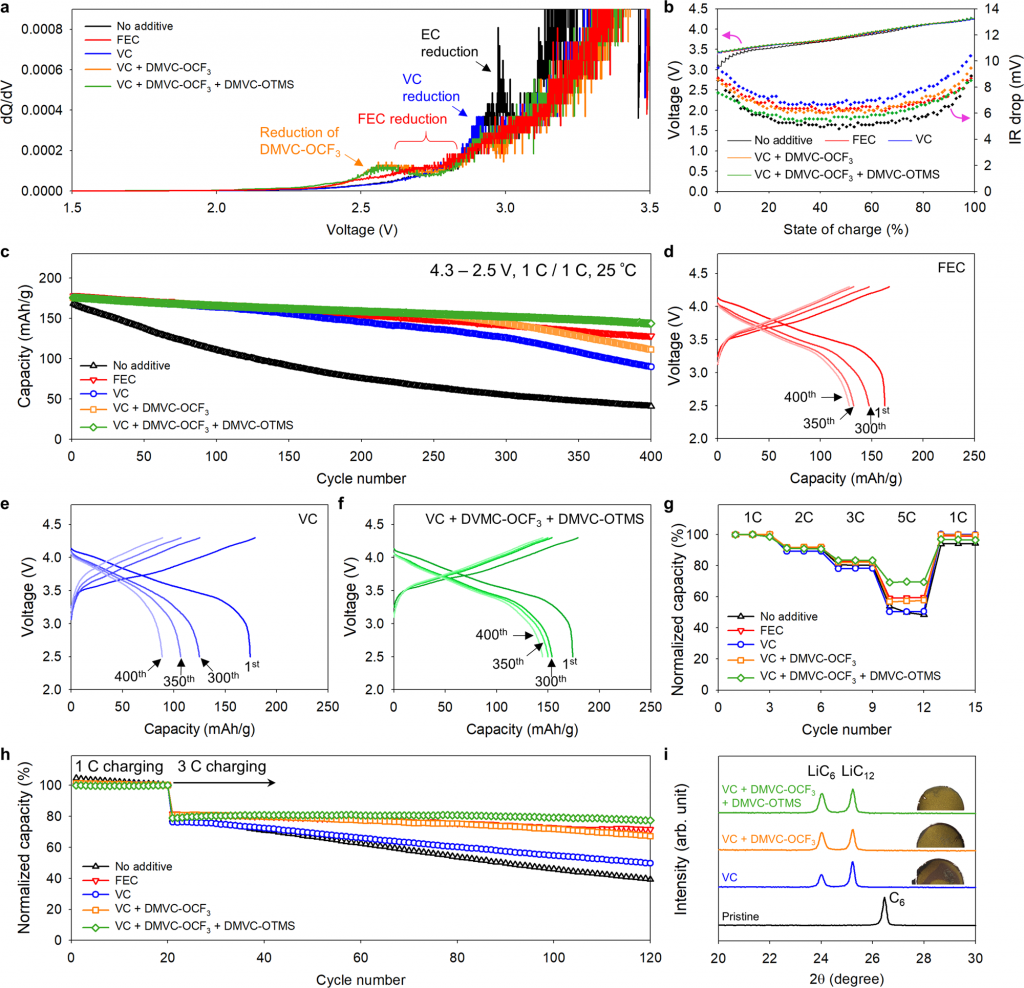
According to the research team, when the new additives were added to a large-capacity battery composed of a high-Ni anodes and a Si-mixed anode, the initial capacity was maintained at 81.5% even after 400 charging and discharging cycles—10% to 30% better than the choice of VC (vinylene carbonate) or FEC (fluoroethylene carbonate), as additive in LIBs.
“This achievement is the result of the collaboration of material structure design, experiment, simulation, and synthesis method research to actually make this material structure that can compensate for the shortcomings of existing additives (VC). It suggested a new direction for the development,” says Professor Choi, co-corresponding author of the study.
Figure 2. Electrochemical performance of synthesized functional additives and fast charging capability.
In addition, the research team also found that these additives could remove hydrofluoric acid (HF) from the electrolyte to prevent the metal (nickel) inside the high-Ni anodes from leaking out. The amount of metal inside the anode determines the battery capacity.
“This work presents a breakthrough in the development of electrolyte additives for high-energy-density Li-ion batteries,” noted the research team. “We expect that our systematic approach for rational molecular design and DFT-aided mechanism development offers a promising way to discover next-generation additives.”
This research has been featured on the Nature Communications Editors’ Highlights webpage. This work has been supported by the Korea Institute of Energy Technology Evaluation and Planning’s energy technology development project. It has also been partly supported by the Technology Development Program to Solve Climate Changes of the National Research Foundation (NRF).
Journal Reference
Sewon Park, Seo Yeong Jeong, Tae Kyung Lee, et al., “Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries,” Nat Commun, (2021).