A research team, affiliated with UNIST has unveiled a cutting-edge catalyst with exceptional oxidizing power, capable of extracting electrons from compounds. Anticipated to revolutionize various fields, including the development of metal catalysts and synthetic chemistry, this catalyst marks a significant breakthrough in catalytic research.
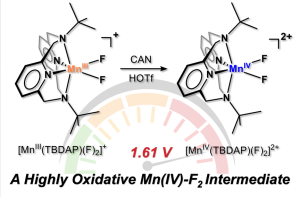
Led by Professor Jaeheung Cho in the Department of Chemistry at UNIST, the research team successfully synthesized the pioneering manganese-fluorine catalyst, utilizing the Macrocyclic Pyridinophane System. This catalyst demonstrates the ability to induce oxidation reactions, facilitating efficient electron loss from toxic toluene derivatives.
Through meticulous analysis, the research team has uncovered the underlying mechanisms responsible for the catalyst’s exceptional performance in oxidation reactions. By modulating the electronic environment of various compounds, the team verified the catalyst’s capability to catalyze the oxidation of toluene derivatives with unparalleled efficiency. This groundbreaking research represents the first exploration of the physicochemical properties of transition metal-fluorine species, introducing a new paradigm for carbon-hydrogen bond decomposition based on electron transfer reactions.
Professor Cho emphasized the significance of activating organic matter with robust carbon-hydrogen bonds, emphasizing their propensity to accept electrons and undergo reduction through high reduction potential chemical reactions. The unique characteristics of manganese-fluorine species enable catalytic transformations in this context.
The advancement of organic catalysts through carbon-hydrogen (C-H) bond activation is a crucial research area with extensive applications in pharmaceuticals and industrial processes. Efforts are underway to develop cost-effective metal catalysts by emulating the activities of diverse metal enzymes through bio-simulation research.
Recent focus has been directed towards metal-halide materials that combine transition metals like iron and manganese with halogen atoms, particularly fluorine, acting as intermediates for oxidizing diverse organic substances. The newly synthesized manganese-fluorine catalyst emerges as the most reactive metal-halide species disclosed to date, offering promising applications in industrial processes.
The research team meticulously analyzed the oxidation mechanism facilitated by the catalyst, showcasing enhanced reaction rates by manipulating the electronic environment of various compounds. Noteworthy is the catalyst’s remarkable efficiency in oxidizing toluene derivatives, a feat previously unseen with existing metal-halide species.
The groundbreaking study, co-authored by researchers Donghyun Jeong and Yujeong Lee under the guidance of Professor Cho, has been published in the online version of the Journal of the American Chemical Society (JACS) on February 4, underscoring its significance in the realm of chemistry. Supported by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT), this research not only propels carbon-neutral technologies forward but also contributes to the advancement of next-generation academics and pivotal progress in environmental and industrial sectors.
Journal Reference
Donghyun Jeong, Yujeong Lee, Yuri Lee, et al., “Synthesis, Characterization, and Reactivity of a Highly Oxidative Mononuclear Manganese(IV)–Bis(Fluoro) Complex,” JACS (2024).