A groundbreaking development in efficient hydrogen storage has been reported by Professor Hyunchul Oh in the Department of Chemistry at UNIST, marking a significant advancement in future energy systems. This innovative research centers around a nanoporous magnesium borohydride structure (Mg(BH₄)₂), showcasing the remarkable capability to store hydrogen at high densities even under normal atmospheric pressure.
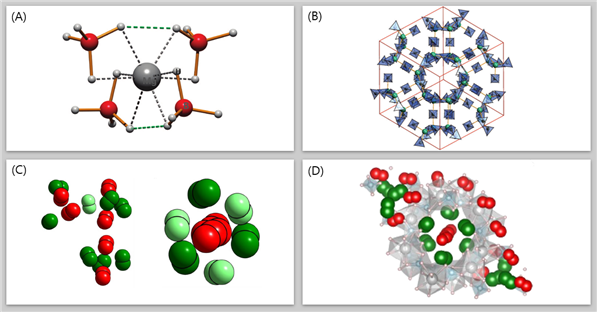
The research team, under the leadership of Professor Oh, has successfully tackled the challenge of low hydrogen storage capacity by leveraging advanced high-density adsorption technology. Through the synthesis of a nanoporous complex hydride comprising magnesium hydride, solid boron hydride (BH4)2, and magnesium cation (Mg+), the developed material enables the storage of five hydrogen molecules in a three-dimensional arrangement, achieving unprecedented high-density hydrogen storage.
The reported material exhibits an impressive hydrogen storage capacity of 144 g/L per volume of pores, surpassing traditional methods, such as storing hydrogen as a gas in a liquid state (70.8 g/L). Additionally, the density of hydrogen molecules within the material exceeds that of the solid state, highlighting the efficiency of this novel storage approach.
Professor Oh emphasizes the significance of this breakthrough, stating, “Our innovative material represents a paradigm shift in the realm of hydrogen storage, offering a compelling alternative to traditional approaches.” This transformative development not only enhances the efficiency and economic viability of hydrogen energy utilization but also addresses critical challenges in large-scale hydrogen storage for public transportation applications.
The study findings have been published ahead of their official publication in the online version of Nature Chemistry, a prestigious international journal in the field of chemistry on February 6, 2024. This research was made possible through the Mid-Career Research Program by the National Research Foundation of Korea (NRF) and the Ministry of Science and ICT (MSIT).
Journal Reference
Hyunchul Oh, Nikolay Tumanov, Voraksmy Ban, et al., “Small-pore hydridic frameworks store densely packed hydrogen,” Nature Chemistry, (2024).